Ferrites for photoelectrochemical water splitting
- Prof. Dr. Detlef Bahnemann, Gottfried Wilhelm Leibniz Universität Hannover, Institut für Technische Chemie
- Prof. Dr. Thomas Bredow, Universität Bonn, Mulliken Center for Theoretical Chemistry, Institut für Physikalische und Theoretische Chemie
- Prof. Dr. Michael Wark, Carl von Ossietzky Universität Oldenburg, Institut für Chemie (IfC), Technische Chemie
The project aims on solar-driven hydrogen formation by photoelectrochemical water splitting employing exclusively abundant elements as electrode materials to find ecologically and economically feasible solutions for future hydrogen production. This requires the optimization of the morphology and the charge collection efficiency of the individual photo-absorber materials for hydrogen and oxygen evolution reactions.
In particular, the project aims at
(i) the development of ferrite, MxFe3-xO4 (M = e.g., Zn, Mg, Ca), based photoelectrodes with enhanced photoresponse utilizing template assisted electrochemical deposition,
(ii) an in depth understanding of the origin and dynamics of charge carriers and reaction intermediates in ferrite based photoelectrochemical cells, and
(iii) the understanding how water and hydroxide ion adsorption on the different crystallographic planes of the ferrites as well as doping and metal substitution affect their band gaps and band positions.
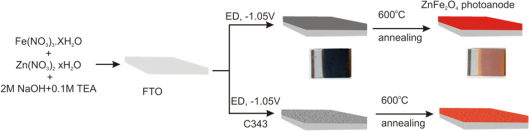
Nanostructured ferrite based electrodes will be produced by template-assisted electrochemical deposition and via screen-printing from nanopowders obtained by different complex-, ionic liquid- and microwave-assisted sol-gel routes. The templates used in this study are small organic molecules with aromatic carboxylic acid functionality imparting porosity and/or specific crystallographic orientation during the deposition of oxide semiconductors due to preferential adsorption at certain crystal planes inducing asymmetric crystal growth. The obtained porosity and crystallographic orientation of the films will govern the photoefficiency of the electrodes. Laser flash photolysis will be used as a powerful tool to probe and elucidate the fate of reaction intermediates and charge transport. In this regard information relating to the lifetime of the charge carriers, the intermediate involved as well as their role in the water splitting reaction will be obtained for both powder and electrode samples. Based on their energetics the morphology and stoichiometry optimized ferrites will be used for photocatalytic Z-schemes and photoelectrochemical cells which will be specially designed for cost-effective water splitting and formation of pure hydrogen in a separated chamber.
An integral part of this project is to gain atomic-scale understanding of the relevant processes using a systematic theoretical approach. By using first-principles methods the mechanisms of the interaction between the various surface planes of ferrite electrodes and water as well as hydroxide ions will be elaborated. The effect of adsorption and M1xM2yFe3-x-yO4 composition of ternary and quaternary phases on the electronic properties (band gap and band alignment) and the surface structure will be obtained employing hybrid DFT in combination with a GW-BSE approach. The application of this DFT/GW-BSE approach to calculate the band gap energy of a semiconductor was recently reported (Lüdtke et al., Inorg. Chem. 53, 2014, 11691-11698).
Photoelectrochemical water splitting and photocatalytic hydrogen production employing this group of compounds has been reviewed (R. Dillert, D. H. Taffa, M. Wark, T. Bredow, and D. W. Bahnemann, Photoelectrochemical water splitting and photocatalytic hydrogen production using ferrites MFe2O4 under visible light irradiation. APL Mater. 2015, 3, in the press [doi: 10.1063/1.4931763].

